How many times has the lack of fire or lack of teeth killed you or someone you love in the wild? Do you dream of successfully following in Captain Sir John Franklin’s footsteps? Then let science be your sextant: learn how to make a battery and avoid scurvy with nothing more than a lemon, copper clips, zinc nails, wire, and steel wool! Watch the video below to find out how.
Electricity is generated through oxidation-reduction, a chemical reaction that occurs when the zinc dissolves into the acid; the lemon itself doesn’t generate electricity, but that doesn’t mean you can’t have your own lemon party!
Scurvy is a disease caused by a vitamin C deficiency whose symptoms include the loss of teeth and eventual death. The word ascorbic acid, the technical term for vitamin C, is derived from scorbutus, Latin for scurvy.
More info: YouTube (h/t: laughingsquid)
You can squeeze about 1 Volt out of a lemon battery
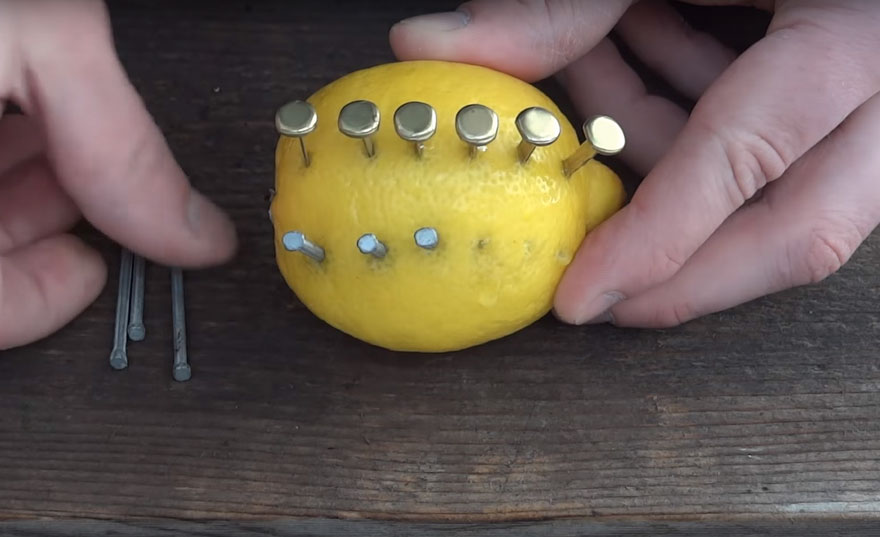
Begin by sticking copper fasteners into the fruit
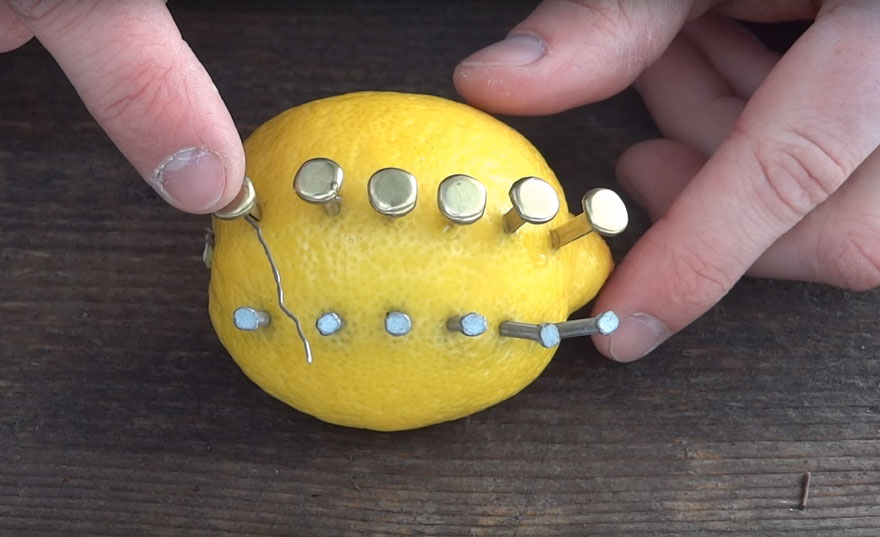
Add some zinc nails
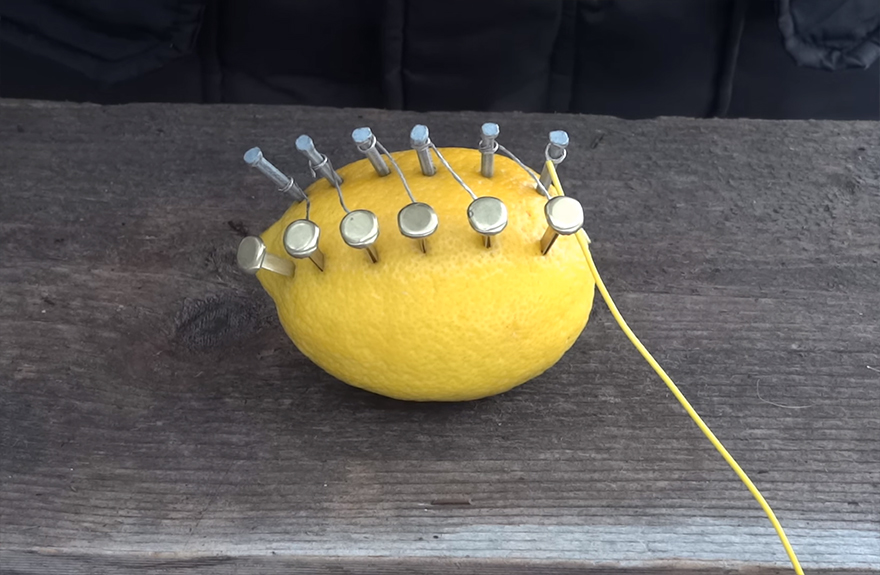
Connect the copper to the zinc on a diagonal
Attach one insulated wire to the free copper fastener and one to the free zinc nail
Touch the two wires to a piece of steel wool to create a short-circuit, and watch the steel wool heat up!
See the full video instructions below:
28Kviews
Share on FacebookAh c**p, no lighter. Thank god I happen to have a lemon, six copper fasteners, zinknails, copper wire and steel wool on me.
That is awesome and very interesting. The only problem is, you'd have to know in advance that you'll be stranded in the woods in order to be sure you bring all the necessary ingredients, lol. ___ What ever happened to the old Scout trick of string, dry sticks and some dry leaves/twigs for tinder?
You can still use those for tinder - but they are not likely going to just light themselves on fire. This shows how to build a lighter.
Load More Replies...Ah c**p, no lighter. Thank god I happen to have a lemon, six copper fasteners, zinknails, copper wire and steel wool on me.
That is awesome and very interesting. The only problem is, you'd have to know in advance that you'll be stranded in the woods in order to be sure you bring all the necessary ingredients, lol. ___ What ever happened to the old Scout trick of string, dry sticks and some dry leaves/twigs for tinder?
You can still use those for tinder - but they are not likely going to just light themselves on fire. This shows how to build a lighter.
Load More Replies...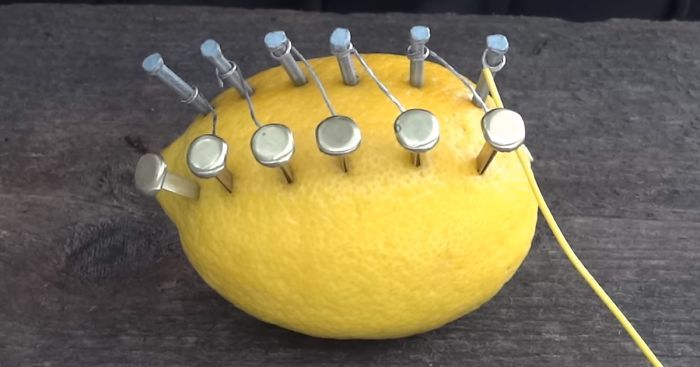






220
23